Review of the best according to the editorial board. On the selection criteria. This material is subjective, does not constitute advertising and does not serve as a purchase guide. Before buying, you need to consult with a specialist.
Our planet is rich in valuable resources, but there are also those whose amount is measured in crumbs. Oddly enough, these elements are some of the most sought after in the world. Among them are heavy metals. Just imagine, an 8-centimeter cube of the heaviest metal in the world weighs as much as 12 kg (!). Today we will focus on the 'heavyweights' in the world of metals.
Top 10 heaviest metals in terms of density
| Nomination | a place | Metal | Density |
| Top 10 heaviest metals in terms of density | 1 | Tantalum | 16.64 g / cm3 |
| 2 | Uranus | 18.92 g / cm3 | |
| 3 | Tungsten | 19.21 g / cm3 | |
| 4 | Gold | 19.85 g / cm3 | |
| 5 | Plutonium | 19.85 g / cm3 | |
| 6 | Neptunium | 20.48 g / cm3 | |
| 7 | Rhenium | 21.01 g / cm3 | |
| 8 | Platinum | 21.44 g / cm3 | |
| 9 | Iridium | 22.53 g / cm3 | |
| 10 | Osmium | 22.62 g / cm3 |
Tantalum
Rating: 4.1

Density: 16.64 g / cm3
Melting / boiling point: 3017 0С / 5458 0С
A very rare metal, but not the heaviest in the world. Under natural conditions, it is a silvery-white solid with a slight bluish tinge (oxide film). It was discovered back in 1802, but it was not immediately possible to isolate it: until 1844 it was identified with another metal – niobium.
Tantalum is one of the most refractory in the world (by this indicator it surpasses even the heaviest metal on the planet) and does not react with air: oxidation of its surface occurs only when the air temperature rises to 280 ° C, which is impossible under natural conditions.
One of the interesting features of tantalum is its paramagnetism (when it enters a magnetic field, the metal is magnetized in the direction of this field). In addition, tantalum is striking in its resistance to aggressive media: its surface does not 'lend itself' even to 70% nitric acid. This unusual metal is used in the military industry (when creating ammunition), medicine (when manufacturing prostheses), in the nuclear industry (when creating nuclear reactors), etc.
An interesting fact: despite its high strength, tantalum is very ductile (it can be compared to gold), so pure metal is very convenient to work with.
Uranus
Rating: 4.2

Density: 18.92 g / cm3
Melting / boiling point: 1132 0С / 3745 0С
The main and not the best characterizing this solid metal difference from other representatives of the rating is its radioactivity. Uranium, being in natural conditions, goes through a long transformation stage, consisting of 14 stages and ending with its transformation into lead. True, this process lasts for billions of years.
In its pure form, uranium has a high weight, silvery-white color, high plasticity (it is slightly softer than steel) and weakly expressed paramagnetic properties. Uranium readily oxidizes upon contact with air, and the powdery substance ignites spontaneously at a temperature of about 150 ° C.
The main and obvious use of uranium is in the nuclear industry. Nuclear energy (production of reactors, power plants, etc.) is considered an active 'consumer' of metal. In recent years, a special stake has been placed on the development of methods for extracting uranium from seawater, where the concentration of solid matter is 3 μg / l).
Tungsten
Rating: 4.3

Density: 19.21 g / cm3
Melting / boiling point: 3422 0С / 3745 0С
It got its rather original name (translated from Latin – 'wolf foam') because, when accompanied by tin ore, it interfered with the smelting of tin, turning it into slag foam. That is, he actually devoured a sheep like a wolf.
Tungsten is a shiny, light gray solid. It is the most refractory metal on the planet: its melting point is close to the solar photosphere. It also has the highest proven boiling point on the planet. True, a 'competitor' has recently appeared – seborgium with a higher (assumed) melting point, but this is not known for certain due to the short duration of the existence of the metal.
At one time, tungsten made a splash in the industry and today it is used as a mandatory basis for heat-resistant alloys. In addition, its high strength provides this metal with wide application in various spheres of human activity: it is used in aircraft engines, filaments, electric vacuum equipment, etc.
Gold
Rating: 4.4
Density: 19.85 g / cm3
Melting / boiling point: 1064 ° C / 2856 ° C
One of the hardest metals on earth, but at the same time it is characterized by incredible plasticity: it can be made of a sheet with a thickness of only 0.1 microns (the so-called gold leaf). It is for this reason that the noble yellow metal has found a worthy place in jewelry. But at the same time, gold has a high density, which greatly simplifies the process of its extraction.
Gold has a very high electrical conductivity, which could make this metal indispensable in the process of creating microcircuits, but alas: the cost of the raw materials is very high, and the prevalence is low.
Gold does not react with oxygen and most elements. The metal does not lend itself to the action of acids and alkalis (an exception is aqua regia, which is used to check the purity of metals). Gold is one of the few metals used not only in industry, but also for the benefit of humans (it is actively used in homeopathy, dentistry). In addition, the noble metal has found active use in banking: it is still a guarantor of the stability of any currency and a reliable investment instrument.
Plutonium
Rating: 4.5

Density: 19.85 g / cm3
Melting / boiling point: 640 0С / 3235 0С
The 'younger brother' of uranium and the possessor of high radioactivity. It is mined under natural conditions, but little and rarely, since it is simply impractical, but it is easy to obtain it in the process of multi-stage conversion of uranium. Became the first chemically artificial substance produced on an industrial scale.
Enriched and natural uranium is used to obtain plutonium. Several years ago, the closure of the world's last plutonium-producing reactor (in Russia) was reported. But that same year, a nuclear reactor was launched in Japan. True, he did not have to work for a long time due to the accident that occurred a couple of months after the launch: the reactor was stopped, and after the tragedy at Fukushima-1, they completely changed their minds to start. In 2016, a decision was made to dispose of the reactor.
Because of the obvious military potential, plutonium began to be actively used in the production of nuclear weapons (the so-called weapons-grade plutonium), as a source of energy for spaceships and as fuel for nuclear reactors.
Neptunium
Rating: 4.6

Density: 20.48 g / cm3
Melting / boiling point: 640 0С / 3235 0С
Another radioactive 'brainchild' of uranium, obtained in the course of nuclear reactions. It is considered the first transuranic element. A relatively soft substance is characterized by good ductility, slowly reacts with air, rapidly oxidizing at its high temperature. On earth, this metal is found in trace amounts, so its extraction in natural conditions is simply pointless.
Neptunium is dangerous to humans during radioactive decay: about 70-80% of its particles settle in the bone tissue, which leads to its complete damage (the degree of damage depends on the valence of the isotopes). Its main application is plutonium production.
Rhenium
Rating: 4.7

Density: 21.01 g / cm3
Melting / boiling point: 3186 ° C / 5596 ° C
The discovery of a dense metal of a silvery color was predicted by Mendeleev back in 1871, and its actual discovery took place only a century and a half later (in 1925). Rhenium became the last among the discovered elements with a stable isotope: all those discovered later did not have such.
Rhenium is one of the rarest elements on our planet. Its geochemical properties are similar to tungsten. The silvery-white metal is considered one of the hardest and densest of all. In its pure form, rhenium is plastic even at room temperature, but at the same time it fully retains its strength even after repeated heating or cooling.
Rhenium is difficult to obtain, and its production is very material-intensive, so the metal is one of the most expensive: the price for 1 kg ranges from 1000 to 10000 dollars. Rhenium 'extraction' occurs primarily in the processing of molybdenum and copper raw materials.
The scope of application of rhenium is due to a number of its properties (refractoriness, resistance to most reagents, etc.). At the same time, its high cost is taken into account: the use of metal is limited to those cases when it gives an advantage over the use of others. Rhenium is mainly used in the manufacture of rocket parts (especially jet and rocket engines).
Platinum
Rating: 4.8

Density: 21.44 g / cm3
Melting / boiling point: 1768 0С / 3825 0С
'Hardy' and solid platinum almost reached the top of our rankings, which is not surprising: it is one of the heaviest metals in the world. The precious substance is also considered one of the rarest on the planet. By the way, even the so-called native metal cannot be considered pure: it contains up to 20% iron, as well as rhodium, iridium, osmium, less often copper.
Platinum is considered one of the most inert metals that does not react with acids and alkalis. The shiny silver metal is actively used in jewelry and glass making, medicine (surgery), the chemical industry, the automotive industry, and due to its resistance to vacuum, it is also used in the creation of spacecraft.
An interesting fact: the majority of the world's platinum reserves are 'hidden' in the depths of only 5 countries – Russia, China, Zimbabwe, South Africa and the United States.
Iridium
Rating: 4.9

Density: 22.53 g / cm3
Melting / boiling point: 2466 0С / 4428 0С
In fact, iridium shares the first place with osmium – the difference in the density of these substances is hundredths of a gram. Nevertheless, this 'heavyweight', nevertheless, is just a little easier. It is a very rare, valuable metal that absolutely does not interact with acids, water and even air. Iridium (like the leader in the ranking of the heaviest metals) is a refractory substance that is difficult to process.
Translated from Greek, it means 'rainbow', which is not surprising, because iridium salts are distinguished by an incredible range of colors: from copper-red to bright blue. White with a light silvery, like a mirror shade, iridium is considered the most durable and one of the rarest on the planet: no more than 10 tons are mined per year, and most of the deposits are located in the place where meteorites fall.
It is used in high-precision engineering as an indicator of the tightness of welded seams. It is actively used by paleontologists and geologists as a temporary indicator of the discovered layer of a particular rock. Often, one of the heaviest metals on the planet is used to generate electricity. In recent years, iridium has received a rather unexpected and unusual application: for electrical stimulation of nerves and in the creation of prostheses for the human eye and ear apparatus.
Osmium
Rating: 5.0
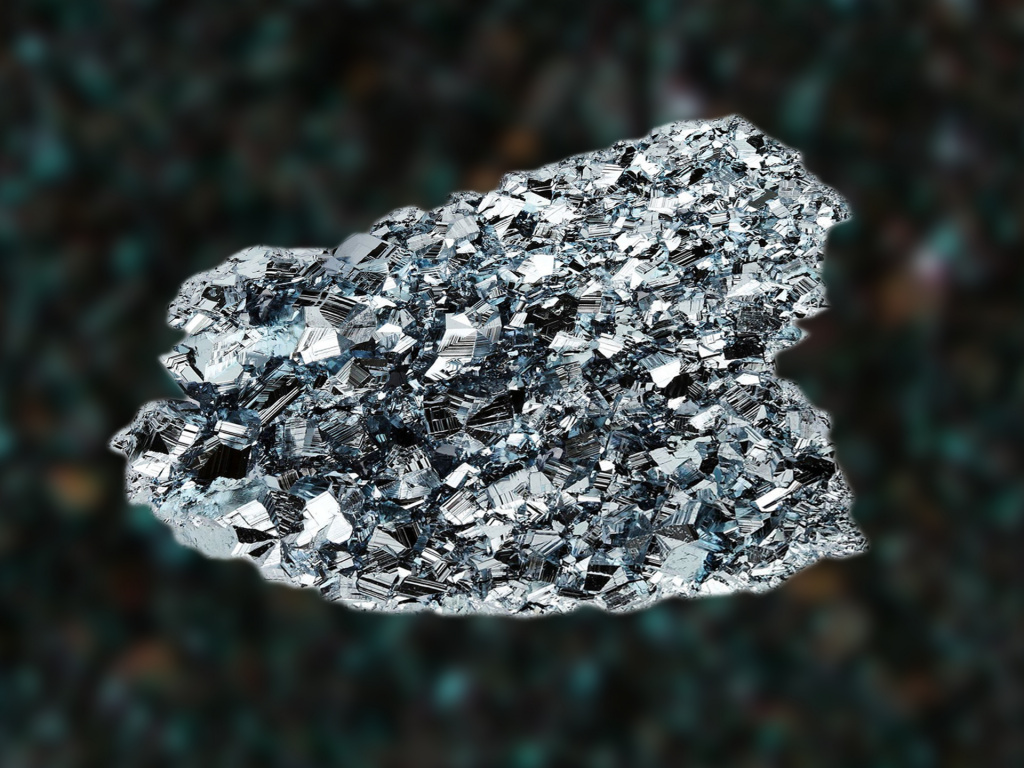
Density: 22.62 g / cm3
Melting / boiling point: 2466 0С / 4428 0С
The heaviest 'representative' of the periodic table, and, accordingly, the heaviest metal in the world. The year 1803 was actually a turning point for this element, since during this period of time its discovery took place literally in racing conditions: two scientists simultaneously discovered osmium – Tennant and de Furcroix. But Tennant, nevertheless, achieved clearer and deeper results, and in official documents filed with the Royal Society of London, he indicated that the element found was conventionally divided into two metals – iridium and osmium.
Osmium is expensive to mine because it is rare and difficult to attack. Hence the impressive cost – $ 15,000 per gram of substance. The density of osmium is only slightly higher than that of iridium, although the properties of both species are not yet fully understood. The heaviest metal in the world is 'unfriendly' to high temperatures: it is very refractory.
Osmium belongs to the group of platinum elements and is conventionally noble. And, although when solidified, osmium forms beautiful silver-blue crystals, it is not suitable for creating jewelry, since it is absolutely non-plastic and difficult to forge. Differs in a specific smell – a garlic-chlorine mixture.
It is highly valued for its strength: the metal is often added to the composition for the manufacture of assemblies subject to frequent and strong friction. Such alloys become incredibly strong and resistant to any impact.
Attention! This rating is subjective and does not constitute an advertisement and does not serve as a purchase guide. Before buying, you need to consult with a specialist.









